Screening
Why screen for autoimmune T1D?
Autoimmune T1D primarily results from the destruction of pancreatic beta cells due to an immune-mediated process, often triggered by environmental factors in individuals who are genetically predisposed.
The risk of developing autoimmune T1D can be assessed by considering family history, including the age of onset and sex of affected family members, along with profiling immunity and genetic markers.1
Who should be screened
Screening for autoimmune T1D is helpful for early detection and management, particularly among high-risk individuals through family history or genetic or immunity markers. This includes:
- First-degree relatives (FDRs) and second-degree relatives (SDRs) of someone with autoimmune T1D, such as a parent, sibling, child, grandparent or cousin1,2
- Patients diagnosed with any autoimmune condition, particularly celiac disease and thyroid disease3
- Those with a family history of autoimmune diseases4
- Patients who have elevated blood sugars and have been previously diagnosed with prediabetes or type 2 diabetes, as they may actually have autoimmune T1D4
Islet autoantibodies in autoimmune T1D
Specific markers in the bloodstream are associated with the development of autoimmune T1D, which includes:*
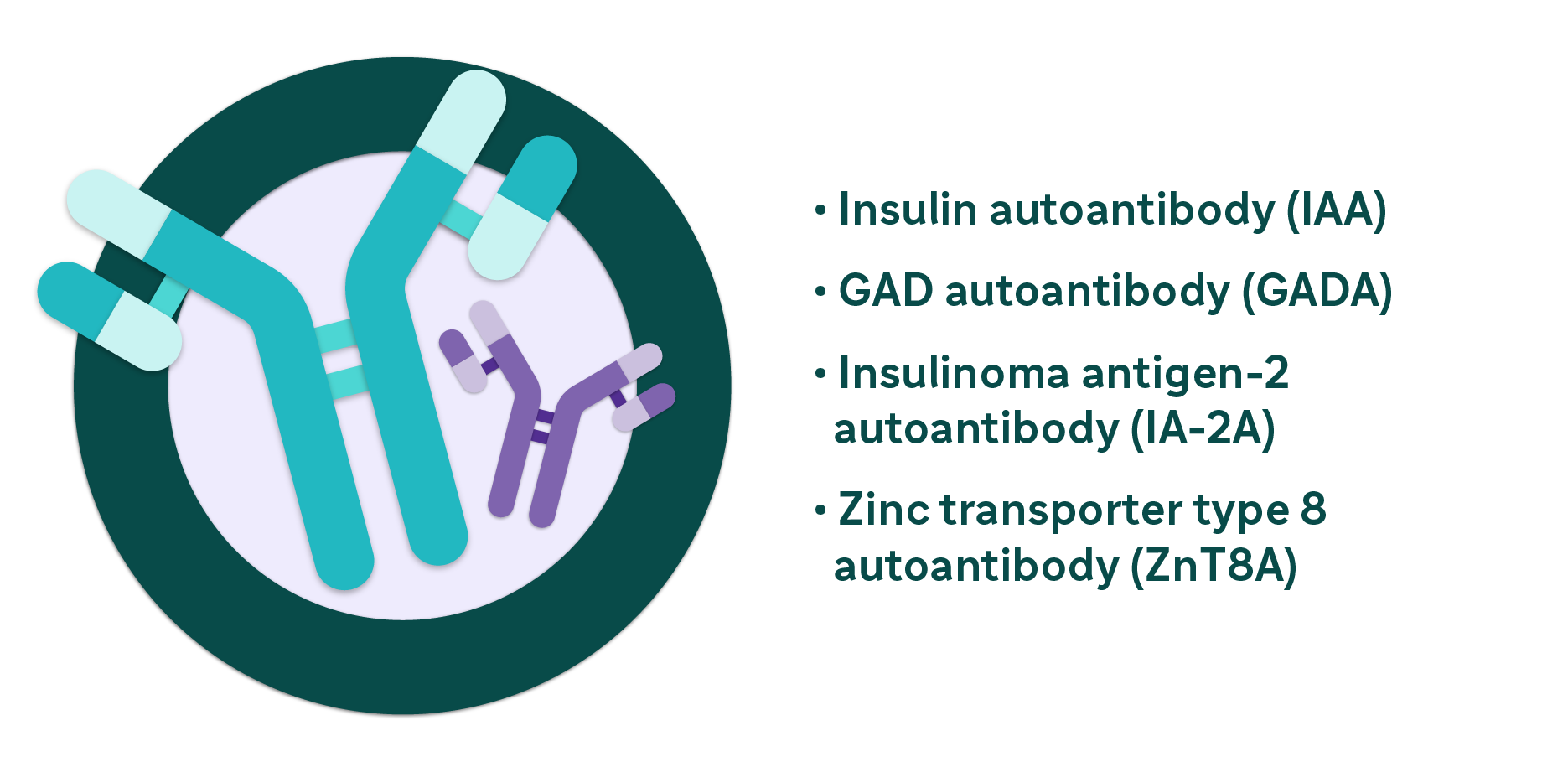
*ICA is also associated with development of autoimmune T1D.
Some of these autoimmune T1D-related autoantibodies can be measured by diagnostic labs or clinics.1,6
Islet autoantibody screening can be performed using a small sample of capillary or venous blood. Several assay methods exist for measuring autoantibodies, and there is variation among methods and the performance of the tests.1,6
When combined with other forms of metabolic testing (glycated hemoglobin test), confirmed autoantibody results are essential for accurately determining the stage of autoimmune T1D.7
Why screening matters

Early detection can identify individuals at risk before severe symptoms appear, allowing for earlier planning and monitoring. This proactive approach may help prevent critical illness and manage the condition more effectively from the outset.8
Incorporating screening for people at risk involves:7,8
- Referral to active screening facilities or research programs when appropriate
- Systematic testing: regularly test all FDRs and other high-risk individuals for autoimmune T1D-associated autoantibodies
- Monitoring those who are in Stage 1 of the disease and referring to an endocrinologist if a positive diagnosis of Stage 2 disease is made
Current screening options available in Canada

Direct patients to sign up for a research program
The TrialNet Research Program provides screening for autoimmune T1D at no cost for relatives of people with autoimmune T1D.9

CanScreenT1D
Breakthrough T1D, in collaboration with Canadian Institutes of Health Research, also announced the launch of CanScreenT1D, a consortium that will explore the feasibility of launching general population screening for early-stage autoimmune T1D in Canada. The pilot program is estimated to start in 2026.10
Currently, general population screening is not recommended in Canada.

Offer screening in your practice at no cost
The UncoverT1D Early Detection Program provides comprehensive 4-autoantibody screening tests at no cost for all individuals, with a focus on those at high risk. This includes individuals who have a personal or family history of aT1D or other autoimmune conditions, or who have elevated blood sugars and may have been previously diagnosed with prediabetes or type 2 diabetes.
Support for you and your patients
The UncoverT1D Early Detection Program is a comprehensive 4-autoantibody testing program designed to help identify eligible individuals at risk of or in the early stages of autoimmune T1D.
The program addresses a critical need for comprehensive and reliable screening in the T1D space, offering:
• First-time screen testing
• Confirmatory testing
• Follow-up testing
This program is designed to facilitate detection of early-stage autoimmune T1D, enabling proactive monitoring and management by HCPs, and support clinicians by providing detailed and accurate testing reports and other resources.
The UncoverT1D Early Detection Program makes it easy to access comprehensive autoantibody testing for screening and monitoring
Monitoring and referral process

Early detection can allow for monitoring, making it easier to prevent critical illness and manage the condition from the outset8.
For individuals who test positive for autoimmune T1D-associated autoantibodies:
- Those in the early stages can be closely monitored for progression. This involves regular follow-ups in accordance with monitoring guidelines.1,11
- Referral to an endocrinologist, where applicable, is recommended11
Read more about screening methods in autoimmune T1D
The DBS method collects blood samples obtained from finger or heel puncturing on a matrix paper, which is subsequently dried.12
Strengths and limitations
DBS sampling has been used for several decades and is comparable to venipuncture results. Sample collection, processing, transportation, and storage are simplified. As DBS samples can be mailed or shipped, the time in between collection and analysis can lead to degradation in quality and interfere with the results.12
The VBS draws venous blood through venipuncture and is analyzed in a lab, with advanced quality control measures.13
Strengths and limitations
This approach delivers more accurate results compared to capillary blood glucose testing, provided the lab adheres to industry standards. The downsides are that the procedure can be painful, it carries a risk of local tissue damage, and it is not ideal for frequent blood sampling.13
Salivary analysis is a non-invasive and convenient alternative to traditional diagnostic methods. Saliva, a complex fluid produced by the salivary glands in the mouth, contains a diverse range of biomolecules, electrolytes, and trace elements that could be used to diagnose an individual’s health.14
Strengths and limitations
The main advantage of salivary glucose monitoring is that it is non-invasive. Saliva can be collected easily and painlessly without the need for needles or lancets. However, there are some limitations: salivary glucose levels can vary due to factors like oral hygiene, oral health, diet, stress, and medication use. Additionally, the lack of a standardized testing method, including the need for further validation in sensitivity, specificity, and calibration, poses challenges. This method is also relatively new and tends to be more expensive.14
Find out more about autoantibody detection methods in autoimmune T1D
RBA uses radioisotopes to detect islet autoantibodies. It employs a radioactively labelled antigen to identify autoantibody binding, measuring the radioactivity to determine the presence and level of autoantibodies.7,15
Strengths and limitations
This method is considered the gold standard assay in autoimmune T1D in terms of accuracy for detecting autoantibodies. However, when applied to a population screening level, it is time-consuming, inefficient, and expensive. The majority of autoantibody positivity were found to be single autoantibodies, which were low risk with low affinity, which translated to minimal disease relevance.15
ECL is a modern, radiation-free technique that detects autoantibodies through light emission from a chemical reaction. It involves two labelled antigens binding to the autoantibody, creating a detectable signal.7,15
Strengths and limitations
ECL is known for its high sensitivity and specificity and can measure multiple autoantibody types in a single test, which has a strong application for population-wide screening. Limitations of ECL include the requirement for a special plate reader.15
ELISA detects autoantibodies using enzymes that attach to antibodies in the blood.7,15
Strengths and limitations
ELISA is used in commercial labs to detect ICA, GADA, IA-2A, and ZnT8A; however, the IAA tests are less sensitive. The sensitivity and specificity seen in known T1D samples are similar to RBA.16
ADAP can be performed on dried blood spot samples. It uses synthetic antigen-DNA conjugates that bind to autoantibodies, forming complexes that allow DNA ligation.7,15,16
Strengths and limitations
The autoantibody levels are quantifiable by qPCR. ADAP can measure multiple islet autoantibodies from a single sample and consistently achieves good sensitivity and specificity. However, as a newer method, further validation is required for non-diabetic population screening on IA-2A and ZnT8A.15
IFA is a cell-based assay that measures serum binding to human islet cells, detecting autoantibodies targeting multiple antigens. Unlike other methods that are specific to single autoantibodies, IFA can identify autoantibodies against several antigens.17
Strengths and limitations
The assay can identify multiple islet autoantibodies. However, ICA is time-consuming, requires human pancreatic tissue, and does not easily yield quantitative results.17
To learn more about which sample collection methods and assays are used as part of the UncoverT1D Early Detection Program, see here.
ICA=islet-cell antibody.
sanofi-aventis Canada Inc. is the sponsor of the UncoverT1D Early Detection Program. Revvity is the sole testing provider. Testing can be ordered at no cost for eligible patients by health care providers. This program is sponsored to address unmet testing needs in the autoimmune T1D early detection space.
The UncoverT1D Early Detection Program is not intended to and should not interfere in any way with a healthcare professional’s or patient’s independent judgment and freedom of choice. Healthcare professionals and patients should always consider the full range of testing and treatment options and select those most appropriate for the individual patient.
Any requests or questions regarding the testing services offered through the UncoverT1D Early Detection Program should be directed to Revvity by email at genomics@revvity.com or by phone at 1-866-354-2910.
The content provided is for informational purposes only and is not a substitute for professional medical advice.
- Ekoe J-M, et al. Screening for Diabetes in Adults. Can J Diabetes 2018;42:S16–9.
- Moore D, et al. Recommendations for screening and monitoring the stages of type 1 diabetes in the immune therapy era. Int J Gen Med 2024;17:3003–14.
- Popoviciu M, et al. Type 1 diabetes mellitus and autoimmune diseases: A critical review of the association and the application of personalized medicine. J Pers Med 2023;13(422):1–20.
- Holt RIG, et al. The Management of Type 1 Diabetes in Adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021;44(11):2589–625.
- Punthakee Z, et al. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can J Diabetes 2018;42:S10–5.
- Phillip M, et al. Consensus Guidance for Monitoring Individuals With Islet Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes. Diabetes Care 2024 Jun 24:dci240042. doi: 10.2337/dci24-0042. Online ahead of print.
- Sims EK, et al. Screening for Type 1 Diabetes in the General Population: A Status Report and Perspective. Diabetes 2022;71:610–23.
- TrialNet. TrialNet Recommendations for Clinicians. Available at: https://www.trialnet.org/healthcare-providers. Accessed July 12, 2024.
- TrialNet. Pathway to Prevention. Available at: https://www.trialnet.org/our-research/risk-screening. Accessed July 12, 2024.
- JDRF. CanScreenT1D: Screening Research Consortium in Canada Announced. Available at: https://jdrf.ca/canscreent1d-screening-research-consortium-in-canada-announced/. Accessed July 12, 2024.
- Besser REJ, et al. ISPAD clinical practice consensus guidelines 2022: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes 2022;23(8):1175–87.
- Mastronardi CA, et al. The use of dried blood spot sampling for the measurement of HbA1c: a cross-sectional study. BMC Clin Pathol 2015;15:3.
- Mathew TK, et al. Blood Glucose Monitoring. [Updated 2023 Apr 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available at: https://www.ncbi.nlm.nih.gov/books/NBK555976.
- Ko A and Liao C. Salivary glucose measurement: A holy ground for next generation of non-invasive diabetic monitoring. Hybrid Advances 2023;3:100052.
- Jia X and Yu L. Effective assay technologies fit for large-scale population screening of type 1 diabetes. Front Clin Diabetes Health 2023;23:3:1034698.
- Ross E and Altimus C. Type 1 Diabetes Autoantibody Screening: A Roadmap for Pediatric Policy Implementation. Milken Institute 2021:1–41.
- Kawasaki E. Anti-Islet Autoantibodies in Type 1 Diabetes. Int J Mol Sci 2023;24(12):10012.
